

News
新闻与媒体
Recently, the shipment ceremony of Dopamine Hydrochloride Injection to the United States was held in Poly Pharmaceuticals' Hainan factory. Trucks loaded with Dopamine Hydrochloride Injection departed for the United States. Following a tornado that struck North Carolina on July 19 and caused severe damage to Pfizer's local factory, Poly Pharmaceuticals rapidly supplied the product, alleviating the shortage of Dopamine Hydrochloride drugs in the United States to some extent. This will have a positive impact on the company's international market expansion.
Poly Pharmaceuticals' Dopamine Hydrochloride Injection received approval from the U.S. Food and Drug Administration (FDA) in December 2022, following its successful development.

Product Details
Drug Name: Dopamine Hydrochloride Injection
• Indications: Used for short-term supportive treatment of heart failure due to decreased myocardial contractility in organic heart disease, including low cardiac output syndrome after cardiac direct visualization surgery.
• Dosage Form: Injection
• Specifications: 20 mL: 250 mg, 40 mL: 500 mg
• Approval Numbers: National Drug Approval Nos. H20237021, H20237022
• Marketing Authorization Holder and Manufacturer: Hainan Poly Pharmaceutical Co., Ltd.
• Associated Active Pharmaceutical Ingredient (API) Name: Dopamine Hydrochloride
• API Registration Number: Y20190004626 (A)
• API Manufacturer: Hainan Poly Pharmaceutical Co., Ltd.
After successfully developing Dopamine Hydrochloride Injection (in specifications of 20 mL: 250 mg and 40 mL: 500 mg), Poly Pharmaceuticals submitted generic drug registration applications to multiple countries and regions. This product sharing same production line with the same standard and same quality.
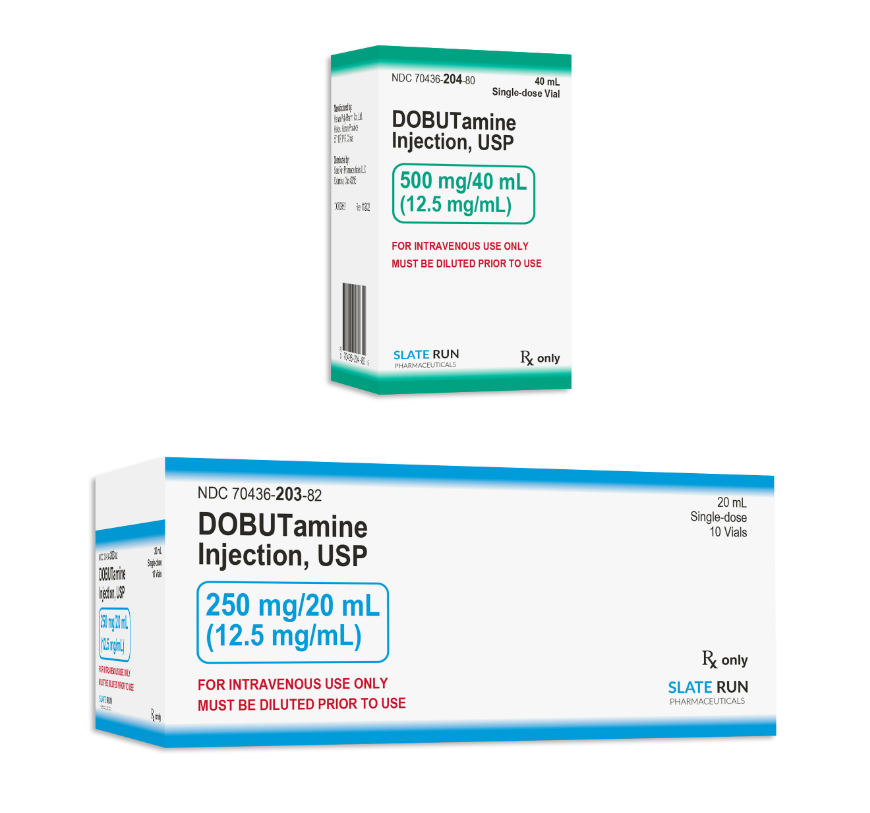
Poly Pharmaceuticals' Dopamine Hydrochloride Injection received approval from the U.S. Food and Drug Administration (FDA) in December 2022 and obtained a Drug Consistency Evaluation Registration Certificate issued by the National Medical Products Administration in March of this year. Poly Pharmaceuticals produces its own Dopamine Hydrochloride API, achieving integration between the API and the finished product.
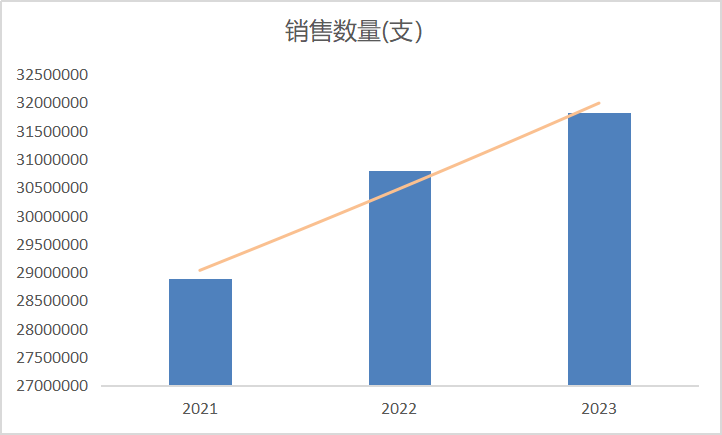
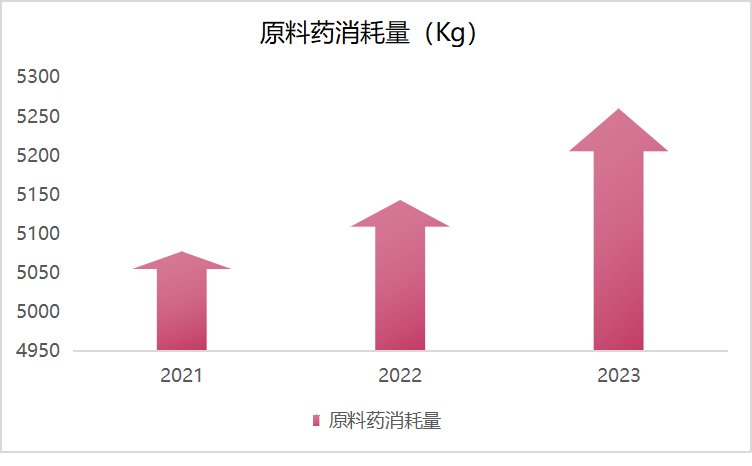
According to IMS data, the global market sales of Dopamine Hydrochloride Injection reached CNY 820 million in 2022, with annual sales of 32 million vials and API consumption of 5.2 tons, showing steady growth. The United States, as the most developed country in healthcare and the second-largest global market for this product and its API, highly recognizes the exported Dopamine Hydrochloride Injection. This signifies the exceptional clinical value of the drug, which is expected to play a significant clinical role in the future.
For inquiries, please contact:
Contact: Mr. Zhou
Email: zm@eagleeyed.com.cn